Menene titanium dioxide?
Babban abin da ke tattare da titanium dioxide shine TIO2, wanda shine muhimmin sinadari na sinadari mai mahimmanci a cikin nau'in farin ƙarfe ko foda. Ba shi da guba, yana da babban fari da haske, kuma ana la'akari da mafi kyawun launin launi don inganta launin fata. Ana amfani da shi sosai a masana'antu kamar su sutura, robobi, roba, takarda, tawada, yumbu, gilashi, da sauransu.

Ⅰ.Jadawalin sarkar masana'antar titanium dioxide:
(1) The sama na titanium dioxide sarkar hada da albarkatun kasa, ciki har da ilmenite, titanium concentrate, rutile, da dai sauransu;
(2)Matsakaici yana nufin samfuran titanium dioxide.
(3) Ƙarƙashin ƙasa shine filin aikace-aikacen titanium dioxide.Ana amfani da titanium dioxide sosai a fannoni daban-daban kamar su rufi, robobi, yin takarda, tawada, roba, da sauransu.

Tsarin crystal na titanium dioxide:
Titanium dioxide wani nau'in fili ne na polymorphous, wanda ke da nau'ikan crystal guda uku a yanayi, wato anatase, rutile da brookite.
Dukansu rutile da anatase suna cikin tsarin kristal tetragonal, waɗanda suke da ƙarfi a ƙarƙashin yanayin zafi na al'ada; Brookite nasa ne na tsarin kristal orthorhombic, tare da tsarin kristal mara ƙarfi, don haka yana da ɗan ƙima mai amfani a masana'antu a halin yanzu.

Daga cikin sifofi uku, lokaci rutile shine mafi kwanciyar hankali. Lokacin Anatase ba zai sake juyewa ba zuwa lokacin rutile sama da 900 ° C, yayin da lokacin brookite zai canza ba tare da juyowa ba zuwa lokacin rutile sama da 650°C.
(1) Rutile lokaci titanium dioxide
A cikin rutile lokaci titanium dioxide, Ti atoms suna tsakiyar tsakiyar lattice crystal, da kuma oxygen atom shida suna located a sasanninta na titanium-oxygen octahedron. Kowane octahedron yana haɗe zuwa 10 kewaye da octahedrons (ciki har da madaidaitan rabo takwas da gefuna na rabawa), da ƙwayoyin TiO2 guda biyu suna samar da tantanin halitta naúrar.
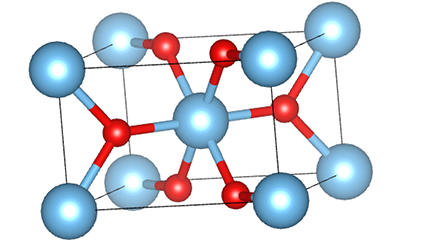
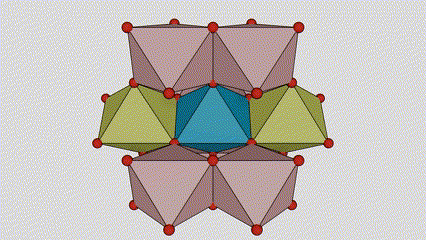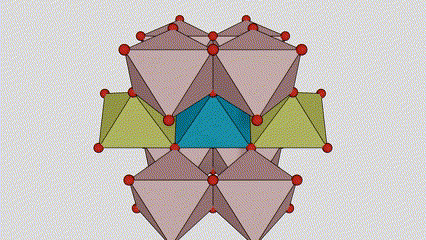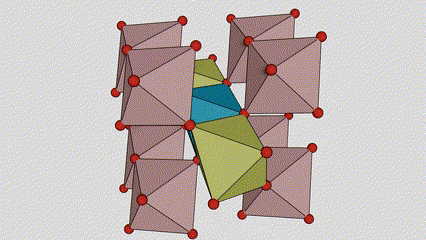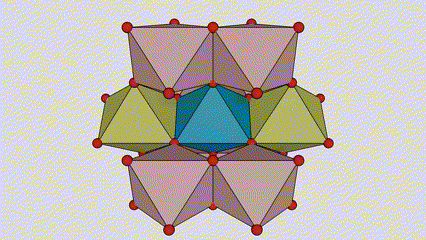
Tsarin tsari na kwayar kristal na rutile lokaci titanium dioxide (hagu)
Hanyar haɗi na titanium oxide octahedron (dama)
(2) Anatase lokaci titanium dioxide
A lokacin anatase titanium dioxide, kowane titanium-oxygen octahedron an haɗa shi zuwa 8 kewaye da octahedrons (raba gefuna 4 da 4 sharing vertices), da 4 TiO2 kwayoyin halitta naúrar cell.
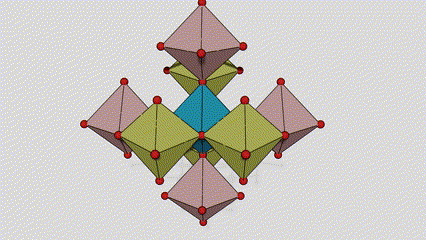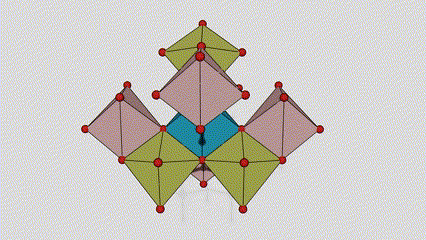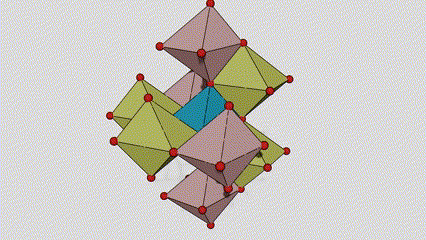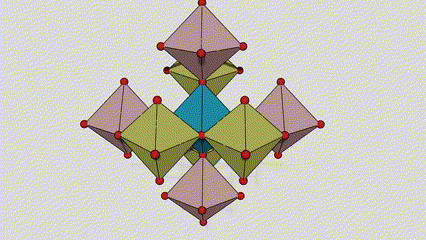
Tsarin tsari na kwayar kristal na rutile lokaci titanium dioxide (hagu)
Hanyar haɗi na titanium oxide octahedron (dama)
Ⅲ.Hanyoyin Shirye-shiryen Titanium Dioxide:
Tsarin samar da titanium dioxide ya ƙunshi tsarin sulfuric acid da tsarin chlorination.

(1) Sulfuric acid tsari
Tsarin sulfuric acid na samar da titanium dioxide ya haɗa da halayen acidolysis na titanium baƙin ƙarfe foda tare da sulfuric acid mai mahimmanci don samar da titanium sulfate, wanda aka sanya shi hydrolyzed don samar da acid metatitanic. Bayan calcination da murkushewa, ana samun samfuran titanium dioxide. Wannan hanya na iya samar da anatase da rutile titanium dioxide.
(2) Tsarin chlorination
Tsarin chlorination na samar da titanium dioxide ya ƙunshi hada rutile ko babban titanium slag foda tare da coke sannan kuma ɗaukar chlorination mai zafi don samar da titanium tetrachloride. Bayan iskar oxygen mai zafi, ana samun samfurin titanium dioxide ta hanyar tacewa, wanke ruwa, bushewa, da murkushewa. Tsarin chlorination na samar da titanium dioxide zai iya samar da samfuran rutile kawai.
Yadda za a bambanta ingancin titanium dioxide?
I. Hanyoyin Jiki:
(1)Hanya mafi sauƙi ita ce kwatanta rubutun ta taɓawa. Titanium dioxide na karya yana jin santsi, yayin da titanium dioxide na gaske ke jin ƙanƙara.

(2)Ta hanyar kurkure da ruwa, idan ka sanya titanium dioxide a hannunka, na karya yana da sauƙin wankewa, yayin da na gaske ba shi da sauƙin wankewa.

(3)Ɗauki kofi na ruwa mai tsabta kuma a zubar da titanium dioxide a ciki. Wanda ke yawo a sama na gaske ne, yayin da wanda ya daidaita zuwa kasa karya ne (wannan hanyar na iya yin aiki ga samfuran da aka kunna ko gyara).
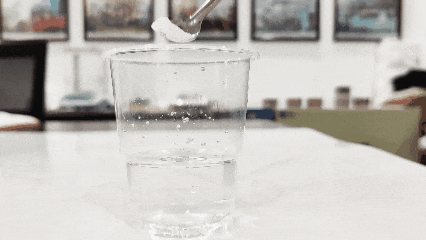

(4)Duba narkewar sa cikin ruwa. Gabaɗaya, titanium dioxide yana narkewa a cikin ruwa (sai dai titanium dioxide da aka kera musamman don robobi, tawada, da wasu titanium dioxide na roba, waɗanda ba sa narkewa cikin ruwa).

II. Hanyoyin sinadarai:
(1) Idan an ƙara foda na calcium: Ƙara hydrochloric acid zai haifar da amsa mai karfi tare da sauti mai tsauri, tare da samar da adadi mai yawa na kumfa (saboda calcium carbonate yana amsawa da acid don samar da carbon dioxide).

(2) Idan an ƙara lithopone: Ƙara sulfuric acid ko hydrochloric acid zai haifar da ruɓaɓɓen ƙamshin kwai.

(3) Idan samfurin shine hydrophobic, ƙara hydrochloric acid ba zai haifar da amsa ba. Duk da haka, bayan jika shi da ethanol sannan kuma ƙara hydrochloric acid, idan an samar da kumfa, yana tabbatar da cewa samfurin ya ƙunshi foda mai rufi na calcium carbonate.

III. Hakanan akwai wasu hanyoyi masu kyau guda biyu:
(1) Ta hanyar yin amfani da wannan tsari na PP + 30% GF + 5% PP-G-MAH + 0.5% titanium dioxide foda, ƙananan ƙarfin abin da aka samu shine, mafi ingancin titanium dioxide (rutile) shine.
(2) Zaɓi guduro mai haske, kamar ABS na gaskiya tare da 0.5% titanium dioxide foda ƙara. Auna isar da hasken sa. Ƙananan watsa hasken shine, mafi ingancin foda na titanium dioxide shine.
Lokacin aikawa: Mayu-31-2024





